Primary cell and Electrolytic cell
Mnemonic
- The positive electrode and cathode are only responsible for transferring electrons
- Primary cell
- Negative oxidation; positive reduction (负氧正还)
- Positive positive negative negative (正正负负): Positively charged ions flow to the positive electrode, and negatively charged ions flow to the negative electrode.
- Positive increase; negative decrease (正增负减): The pH of positive electrode increases, and the pH of negative electrode decreases. Requirements: no proton-exchange membrane
- Negative electrode: Reducing substances For example: metal, \( H_2 \)
- Positive electrode: Oxidizing substances For example: \( O_2 \) \( Cl_2 \)
- Electrolytic cell
- Anode oxidation; cathode reduction (阳氧阴还)
- The anode is connected to the positive pole, and the cathode is connected to the negative pole
- Anode: producing oxidizing substances
- Cathode: producing reducing substances
- The anode and the cathode attract each other (阴阳相吸): Positively charged ions flow to the cathode, and negatively charged ions flow to the anode.
- The cathode rise and the anode fall (阴盛阳衰): The pH of the cathode increases, and the pH of the anode decreases. Requirements: no proton-exchange membrane
NCEE
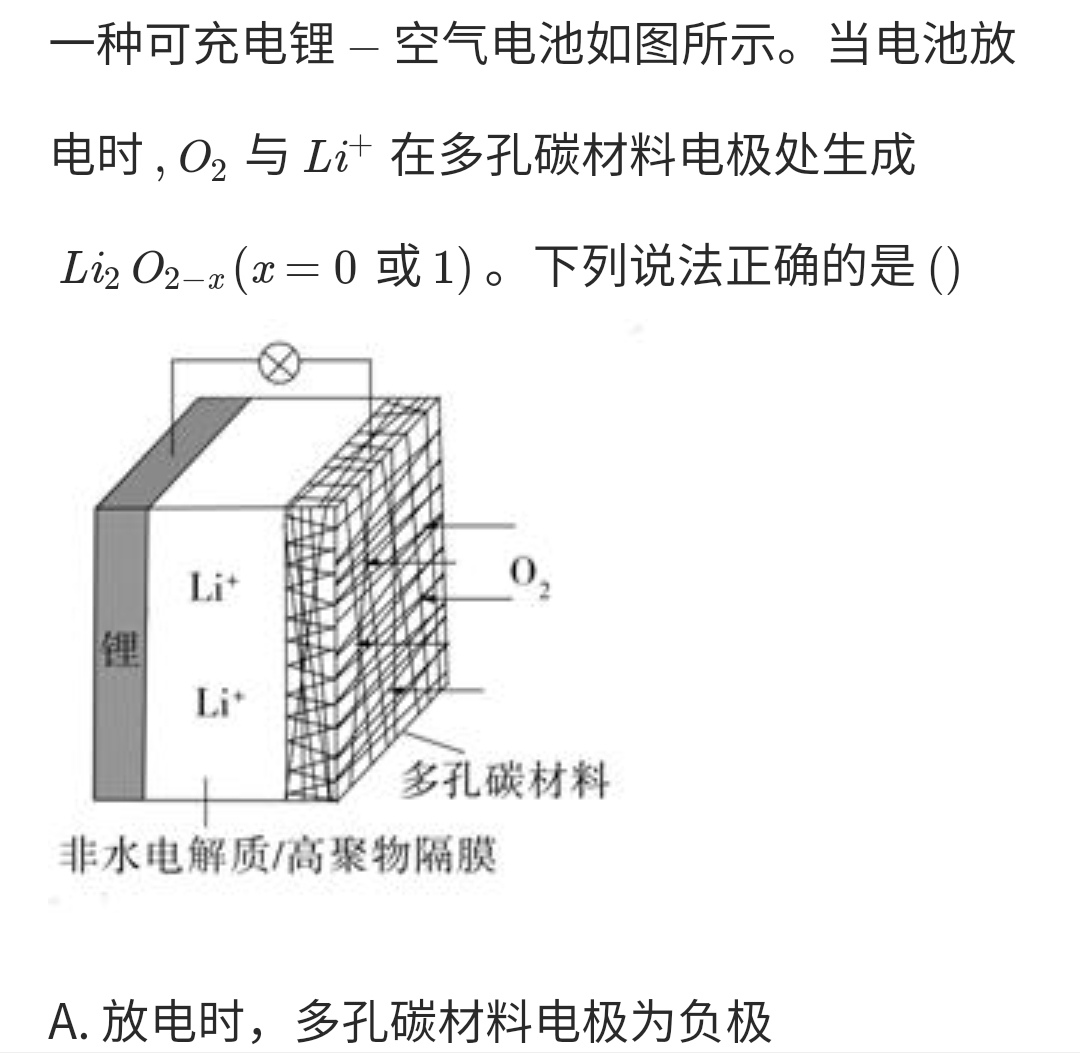
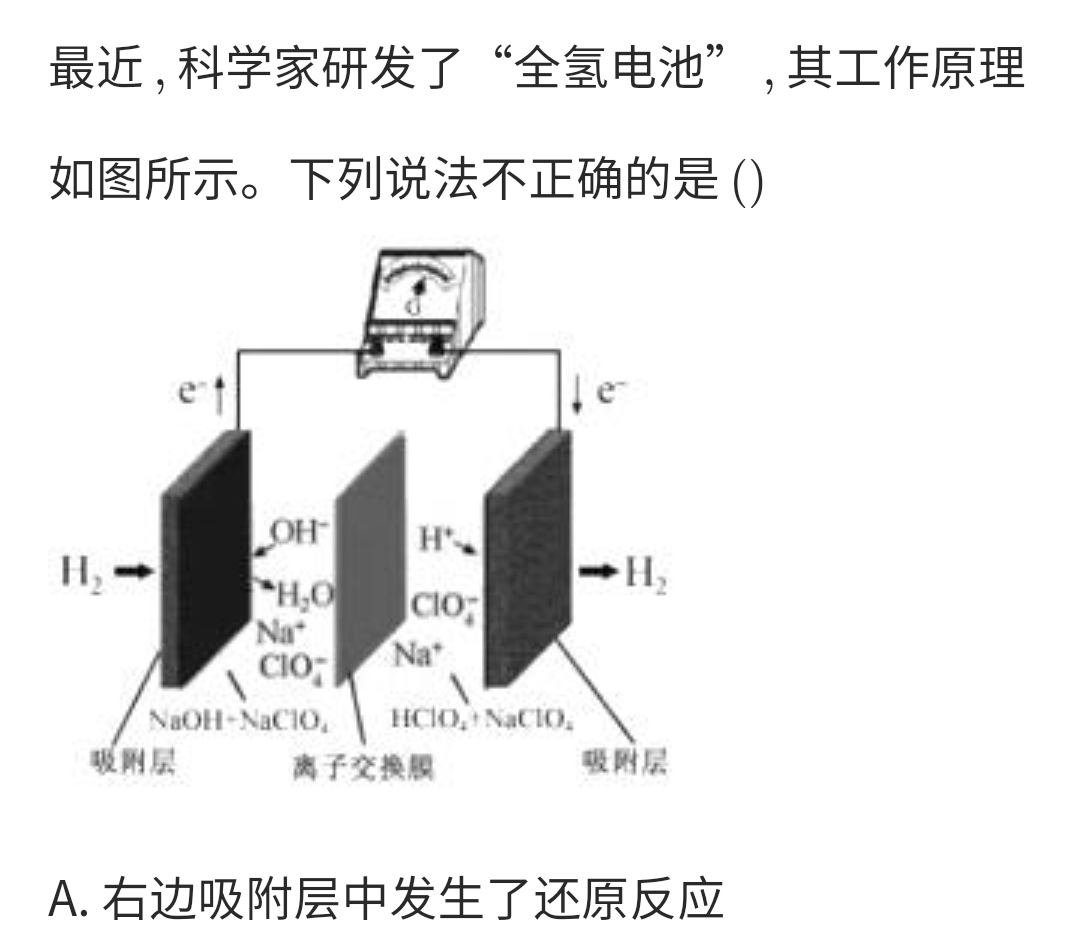
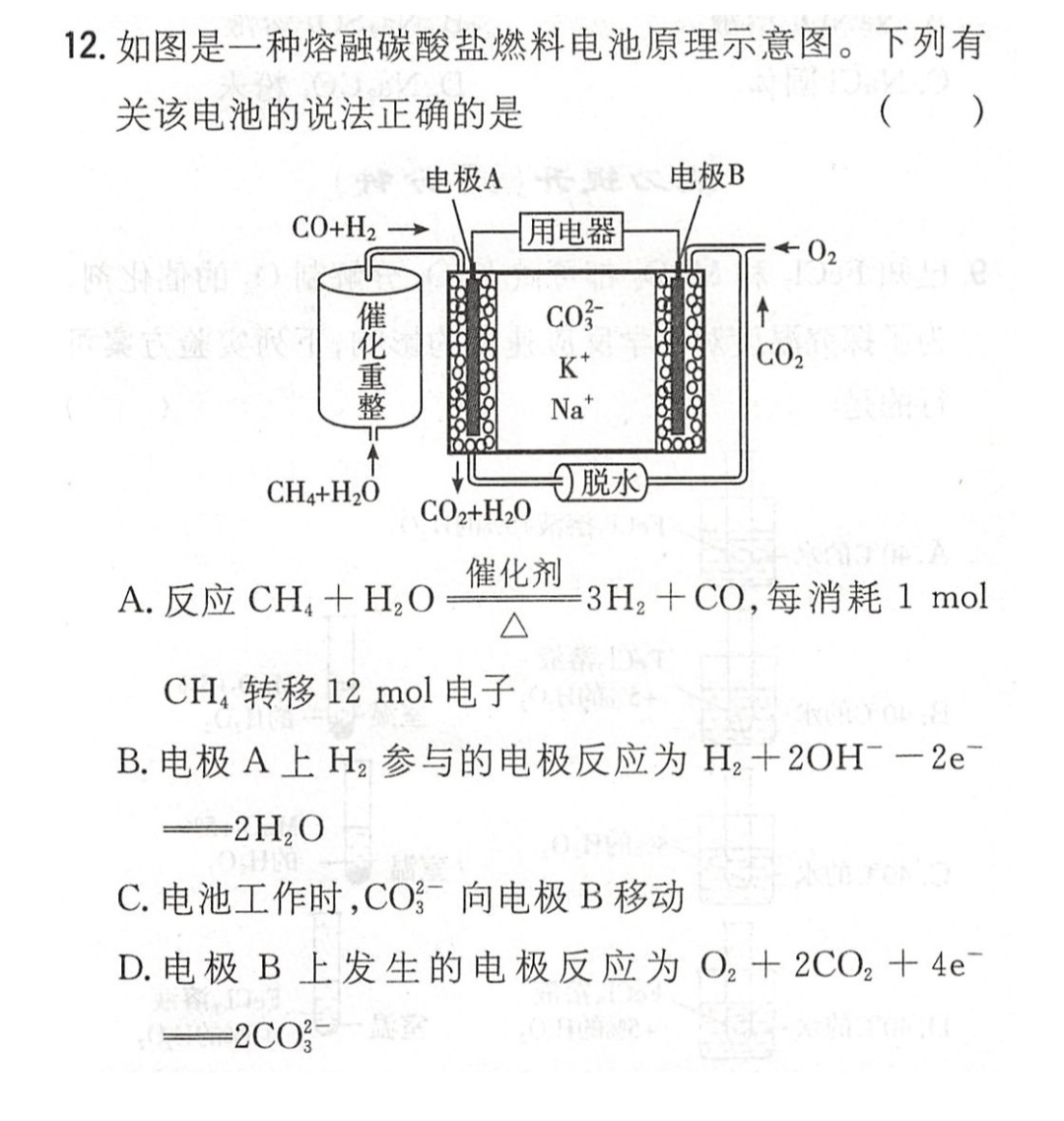
Primary cell
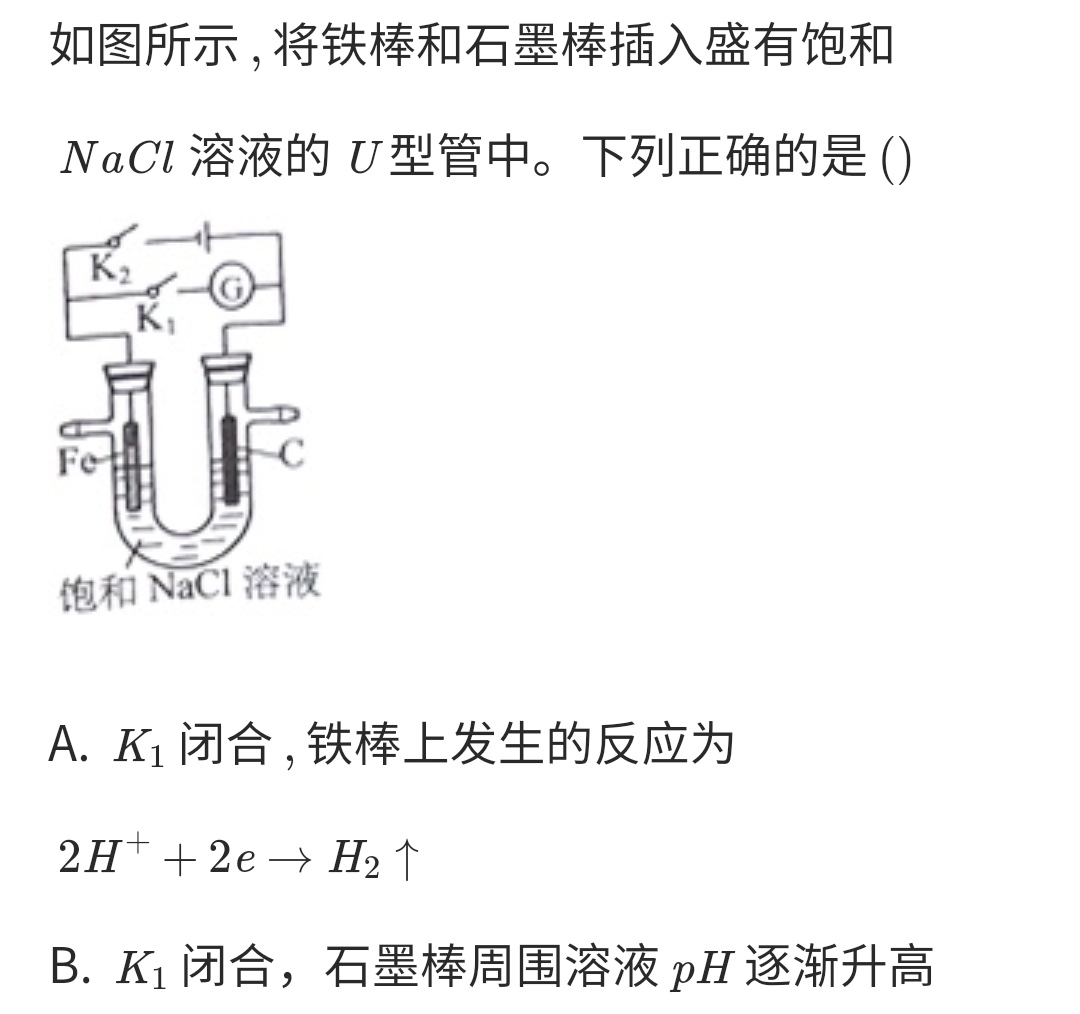
Electrolytic cell

Electrode reaction
